DOPRAM-V- doxapram hydrochloride injection
Boehringer Ingelheim Vetmedica, Inc.
----------
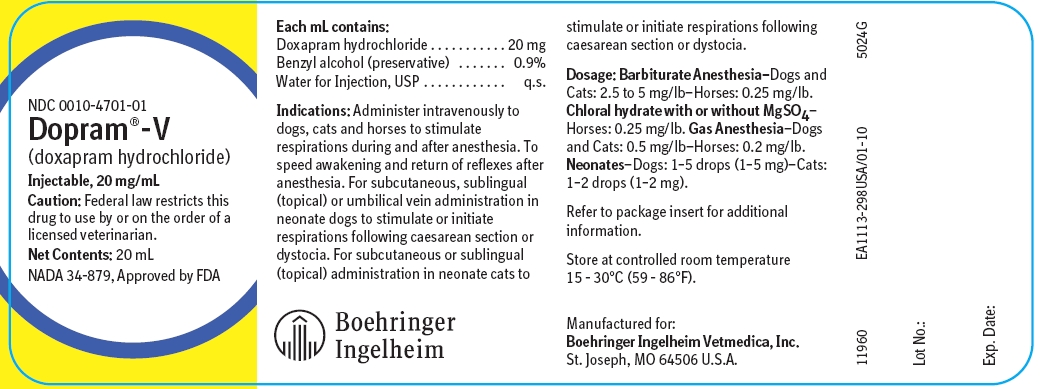
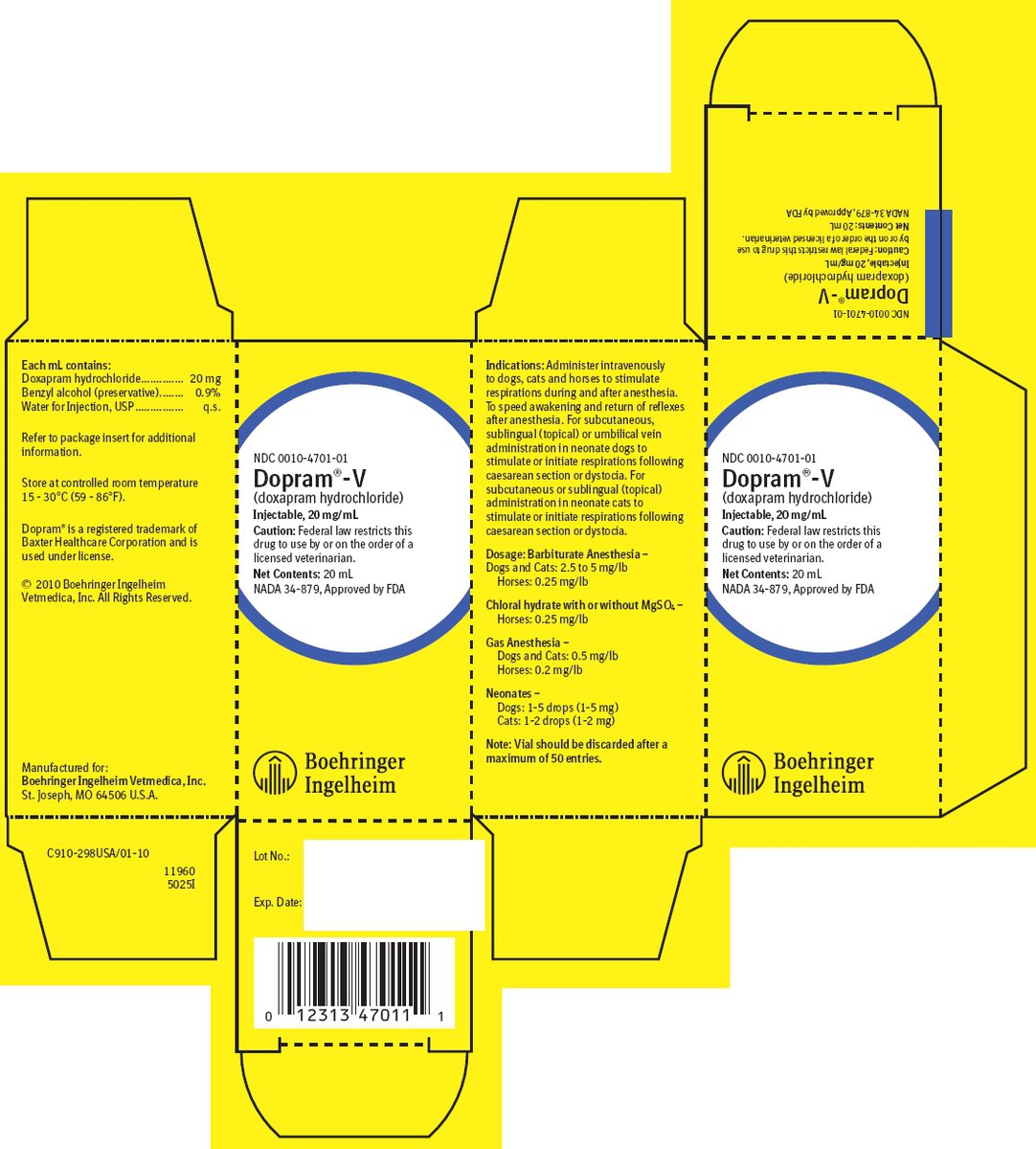
Each 1 mL contains:
Doxapram hydrochloride ............................ 20 mg
Benzyl alcohol (as preservative) ................. 0.9 %
Water for Injection, USP ............................. q.s.
Description
Dopram-V (doxapram hydrochloride) is a potent respiratory stimulant. It is unique in its ability to stimulate respiration at dosages considerably below those required to evoke cerebral cortical stimulation. In nonanesthetized animals the dose required to produce convulsions is some 70 to 75 times the dose required to produce respiratory stimulation. In anesthetized subjects, doxapram also exerts a marked arousal effect. Thus, by promoting the restoration of normal ventilation and producing early arousal following general anesthesia, doxapram minimizes or prevents the undesirable effects of post-anesthetic respiratory depression or hypoventilation and hastens recovery.
Chemistry1
The chemical name of doxapram hydrochloride is 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone hydrochloride hydrate.
The material is prepared as a clear, colorless, 2% aqueous solution with a pH of 3.5 to 5 and is stable at room temperature. Stability studies of 24 months’ duration have shown doxapram to have excellent stability characteristics. The preservative is benzyl alcohol, 0.9% and sterilization is accomplished by aseptic filtration technique. Doxapram is compatible with 5% and 10% dextrose in water or normal saline, but is physically incompatible with alkaline solutions, such as 2.5% thiopental sodium.
Species Variation2,5
The dog responds more dramatically to doxapram than other species. For example, arousal was not observed in the rat, and the cat responded poorly in comparison with the dog. Respiratory stimulation was slight in the rat, moderate in the cat and marked in the dog and horse.
Effect on EEG3
Studies show that while the drug acted selectively on respiratory centers of the brain, higher doses stimulated other parts of the neuraxis. The cortex appeared to be the most resistant part of the central nervous system to the action of the drug.
Effect on Cerebral Blood Flow4
The effect of doxapram on cerebral blood flow in anesthetized dogs was determined. Initially, the drug caused a transient increase in blood flow concomitant with rising femoral arterial blood pressure. Flow then diminished while the blood pressure remained elevated. The decreased flow appeared to coincide with marked respiratory stimulation; its occurrence, therefore, is consistent with the known vasoconstrictor effect of hypocapnia.
Effect on Pituitary-Adrenal Axis5
Intravenous administration of doxapram (20 mg/kg) to anesthetized dogs resulted in a marked rise in the adrenal venous blood level of 17-hydroxycorticosteroids. The peak response occurred at 5–7 minutes in most animals. Hypophysectomy prevented this effect of doxapram.
Site and Mechanism of Action2,3,4,7
Doxapram appeared to stimulate respirations primarily by an effect on the brain stem, since sectioning of reflex pathways did not abolish its action. The detection of increased electrical activity in both the inspiratory and expiratory centers of the medulla, at doses as low as 0.2 mg/kg, constituted confirmation of this site of action. Only after higher doses were other parts of the brain and spinal cord stimulated. Also, cross circulation experiments have shown that doxapram acts mainly through direct stimulation of central respiratory centers.
The pressor response to doxapram appears to be primarily due to stimulation of brain stem vasomotor areas and it is mediated through the sympathoadrenal system. Adrenalectomy and/or drugs which inhibit transmission at sympathetic ganglia or at sympathetic neuroeffector sites were capable of reducing the pressor response to doxapram. Spinal section at C2 abolished the pressor effect.
Intravenous infusion of doxapram to dogs resulted in a prompt and marked increase in total blood and urinary catecholamines.
Therapeutic Ratio2
Doxapram did not produce convulsions as readily as did other respiratory stimulants. In unanesthetized animals the ratios between convulsant and respiratory stimulant doses of several such drugs were as follows: doxapram, 70; ethamivan, 35; bemegride, 15; pentylenetetrazol, 4; and picrotoxin, 2.3. In animals anesthetized with barbiturates, it was not possible to establish this ratio for doxapram because convulsions could not be produced.
Interaction with Other Drugs2,5,10,11,13
The respiratory stimulant effects of doxapram in dogs were not blocked by anesthetic doses of the following: phenobarbital sodium, pentobarbital sodium, thiopental sodium, secobarbital sodium, halothane and methoxyflurane. In dogs and cats, doxapram stimulated respiration that was severely depressed with morphine or meperidine. However, convulsions occurred in cats, a species in which morphine is known to be convulsant.
The respiratory stimulant effects of doxapram in horses were not blocked by anesthetic doses of the following: chloral hydrate, chloral hydrate plus magnesium sulfate and pentobarbital sodium.
Nialamide potentiated the respiratory stimulant action of doxapram in dogs and reserpine suppressed this action. In curarized dogs, the respiratory response varied inversely with the degree of muscle relaxation existing at the time doxapram was administered.
Doxapram antagonized the depressant effects of chlorpromazine, mephenesin and methocarbamol on spinal reflexes in unanesthetized cats.
Various combinations of analeptics in acute barbiturate narcosis in dogs have been compared, including metaraminol and phenylephrine, methetharimide and amphetamine, methetharimide and phenylephrine, pentylenetetrazol and phenylephrine, pentylenetetrazol and amphetamine, doxapram and phenylephrine, and doxapram alone. While most combinations improved respiratory minute volume quickly, doxapram gave the best response of all. In a similar study comparing the effects of doxapram and various analeptic combinations in dogs, only doxapram was conspicuously effective in increasing ventilation and in shortening sleeping time.
Absorption, Distribution and Fate5,8
Respiratory stimulation was observed in the anesthetized dog after administration by the following routes: intravenous, intramuscular, intraperitoneal, oral, sublingual and subcutaneous.
Spectrophotometric methods were applied to the determination of blood levels and urinary excretion in dogs given doxapram, 10 mg/kg and 20 mg/kg, intravenously. Blood concentrations of doxapram and/or its metabolites were at peak levels immediately after injection and declined rapidly in the first hour. The concentration then further decreased slowly, and an appreciable amount was still present at the end of 24 hours. One dog was given doxapram labeled with radioactive carbon in the 2-position of the pyrrolidinone ring. Blood levels were slightly higher and urinary excretion was slightly lower by isotope assay than by chemical assay. The feces contained 29% of the administered radioactivity after 48 hours and an additional 9% in the following 24 hours.
Animal Toxicology5,9
Oral toxicity studies were carried out in nine dogs and sixty rats for 30 days. Dogs were given doxapram orally by capsule at doses of 20, 50 and 125 mg/kg/day, and one group received the drug intravenously at 20 mg/kg/day. Rats received the drug by stomach tube at 40, 80 and 160 mg/kg/day, with one group receiving 20 mg/kg intravenously daily. Four dogs died, three while receiving the high dose of 125 mg/kg and one at 50 mg/kg. At each dosage level signs of tremor, lacrimation, excessive salivation, occasional vomiting, diarrhea, stiffness of the extremities and respiratory stimulation were observed in all dogs. The hemogram, urinalysis and blood chemistry showed no changes which were considered attributable to the drug.
Histologically, the central nervous system in both species showed congestion, perivascular hemorrhages and petechial hemorrhages. These changes were interpreted as resembling hypoxic changes. The experiments were repeated in dogs at 2.5, 5, 10 and 20 mg/kg/day and no such lesions were seen.
The acute LD50 of doxapram appears to be in the same dose range for various species of animals including mice, rats, adult dogs, newborn dogs and cats. The intravenous LD50 was approximately 75 mg/kg while the oral and subcutaneous LD50’s were three to four times greater and the intraperitoneal LD50 about twice as great.
No significant irritation was produced when a saline solution of doxapram at a pH of 4.3 was administered intramuscularly to rabbits at concentrations of 1, 2 and 4%. On the other hand, aqueous solutions of the same concentrations caused tissue irritation in rabbits when given subcutaneously.
Safety Margin for the Various Species2,9
The acute LD50 of doxapram HCl in unanesthetized animals appears to be in the same dose range for various species of animals including mice, rats, adult and neonatal dogs and cats. Intravenously, the LD50 was determined to be approximately 75 mg/kg. The oral and subcutaneous LD50 was three to four times the intravenous LD50 whereas the intraperitoneal LD50 was about twice as great.2,9
The maximum tolerated dose (MTD) of doxapram HCl in unanesthetized animals appears to be in the same dose range for various species of animals including mice, rats, adult and neonatal dogs and cats. Intravenously, the highest MTD tested was determined to be approximately 40 mg/kg. The oral and subcutaneous MTD was three or four times the intravenous MTD whereas the intraperitoneal MTD was about twice as great.
The highest dose given intravenously to horses was 66 mg per 100 lbs with chloral hydrate anesthesia, and 60 mg per 100 lbs with gas anesthesia. All animals responded normally and no toxic symptoms were observed.
Clinical Studies5,12,14
The clinical use of doxapram in lightly and deeply anesthetized animals has confirmed the respiratory stimulant and arousal effects previously demonstrated in the laboratory. In one study with 48 dogs and 18 cats subjected to various surgical procedures using pentobarbital sodium as the anesthetic, marked increases in ventilation occurred within one minute following a single intravenous injection of 5 mg doxapram per kg of body weight (2.5 mg/lb). The most dramatic improvement occurred in lightly anesthetized dogs pretreated with either promazine or fentanyl-droperidol and atropine. Doxapram accelerated the return of pedal reflexes in all animals.
Doxapram consistently sustained an increased heart rate beginning one minute after injection. A second injection generally failed to further increase heart rate. EKG disturbances of T-wave polarity and magnitude occurred with the use of doxapram but tended to abate with time. Second injections of doxapram generally did not aggravate the EKG distortions.
Ten animals had pre-existing EKG signs of cardiac damage and tolerated doxapram well.
In another study with 73 dogs subjected to various surgical procedures using methoxy-flurane or halothane as the anesthetic, the arousal time was materially shortened, and respiratory minute volume and rate were increased following a single intravenous injection ranging from 0.08 to 1.95 mg/lb with an average dose of 0.44 mg/lb.
Doxapram was effective in intravenous dosages of 1 mg/kg or less in increasing ventilation and reducing arousal time, especially following methoxyflurane. Tidal volume and respiratory rates were increased; the response normally occurred in 10–20 seconds following injection. No side effects were observed. There were 35 dogs under halothane and 33 dogs under methoxyflurane anesthesia in this study.
In 20 horses subjected to various surgical procedures using intravenous injections of chloral hydrate, chloral hydrate and magnesium sulfate, or pentobarbital as the anesthetic, marked increases in ventilation occurred within 30 seconds following intravenous injection of doxapram in doses ranging from 0.20 to 0.66 mg/lb with an average of 0.28 mg/lb for chloral hydrate and 0.20 to 0.25 mg/lb for the barbiturate. The arousal time was materially shortened, and respiratory minute volume and rate were increased.
In another study involving 34 horses anesthetized with halothane or methoxyflurane, marked increases in ventilation occurred within 30 seconds following intravenous injection of doxapram in doses ranging from 0.08 to 0.50 mg/lb, with an average dose of 0.21 mg/lb. The average recovery time was shortened by one-third or more.
In a series of clinical studies involving 80 neonatal canine patients, suffering respiratory crisis following dystocia or caesarean section, doxapram administered either subcutaneously, sublingually or via umbilical vein in doses from 1–5 drops (1–5 mg) resulted in a marked increase in ventilation and survival of all patients.
In a series of clinical studies involving 16 neonatal feline patients, suffering respiratory crisis following caesarean section or dystocia, doxapram administered either subcutaneously or sublingually (topically) in doses of 1 to 2 drops (1–2 mg) resulted in a marked increase in ventilation and survival of all patients.
Indications
For Dogs, Cats and Horses:
1. To stimulate respirations during and after general anesthesia.
2. To speed awakening and return of reflexes after anesthesia.
For Neonate Dogs and Cats:
1. Initiate respirations following dystocia or caesarean section.
2. To stimulate respirations following dystocia or caesarean section.
Caution: For intravenous use only in dogs, cats and horses. May be administered subcutaneously, sublingually (topically) or via umbilical vein in neonatal puppies and either subcutaneously or sublingually (topically) in neonatal kittens. Do not mix with alkaline solutions. Dopram-V (doxapram hydrochloride) is neither an antagonist of muscle relaxant drugs nor a specific narcotic antagonist.
Doses of Dopram-V should be adjusted to meet the requirements of the situation. Excessive doses may produce hyperventilation which may lead to respiratory alkalosis. A patent air passageway is essential. Adequate, but not excessive, doses should be used and the blood pressure and reflexes should be checked periodically.
|
DOSAGE OF DOPRAM-V (DOXAPRAM HYDROCHLORIDE) FOR INTRAVENOUS INJECTION |
||
|
Dogs and Cats |
||
|
Weight of Animal (lb) |
Barbiturate Anesthesia |
Gas Anesthesia |
|
10 |
1¼ ml (25 mg) to 2 ½ mL (50 mg) |
¼ mL (5 mg) |
|
20 |
2 ½ mL (50 mg) to 5 mL (100 mg) |
½ mL (10 mg) |
|
30 |
3¾ mL (75 mg) to 7½ mL (150 mg) |
¾ mL (15 mg) |
|
50 |
6¼ mL (125 mg) to 12½ mL (250 mg) |
1 ¼ mL (25 mg) |
|
Dosage should be adjusted for depth of anesthesia, respiratory volume and rate. Dosage can be repeated in 15 to 20 minutes, if necessary. |
||
|
Horses |
||
|
Weight of Animal (lb) |
Chloral hydrate, chloral hydrate and magnesium sulfate barbiturates, use 0.0125 mL (0.25 mg) per lb body weight |
Inhalation anesthesia halothane, methoxyflurane use 0.01 mL (0.20 mg) per lb body weight |
|
100 |
1 ¼ mL (25 mg) |
1 mL (20 mg) |
|
200 |
2 ½ mL (50 mg) |
2 mL (40 mg) |
|
500 |
6 ¼ mL (125 mg) |
5 ml (100 mg) |
|
1000 |
12 ½ mL (250 mg) |
10 mL (200 mg) |
DOSAGE OF DOPRAM-V (DOXAPRAM HYDROCHLORIDE) FOR NEONATE USE
Neonate Canine
Doxapram may be administered either subcutaneously, sublingually (topically) or via the umbilical vein in doses of 1 - 5 drops (1 - 5 mg) depending on size of neonate and degree of respiratory crisis.
Technique for Umbilical Vein Administration
When the neonate is presented through the incision of the uterus, placental membrane and fluid are removed from mouth and nose. A clamp is placed across the umbilical cord approximately 1-2 inches from abdomen of neonate. The umbilical vein is isolated and the selected dose of doxapram injected directly into the umbilical vein.
Neonate Feline
Doxapram may be adminstered either subcutaneously or sublingually (topically) in a dose of 1 - 2 drops (1 - 2 mg) depending on severity of respiratory crisis.
Administration and Dosage
The action of Dopram-V (doxapram hydrochloride) is rapid, usually beginning in a few seconds. The duration and intensity of response depends upon the dose, the condition of the animal at the time the drug is administered, and depth of anesthesia. Repeated doses should not be given until the effects of the first dose have passed and the condition of the patient requires it.
Dosage should be adjusted for depth of anesthesia, respiratory volume and rate. Dosage can be repeated in 15 to 20 minutes, if necessary.
Note: Vial should be discarded after a maximum of 50 entries.
How Supplied
Dopram-V (doxapram hydrochloride) is available in 20 mL multiple dose vials of the sterile solution.
NDC 0010-4701-01 - 20 mL multiple dose vial - 20 mg/mL
Bibliography
1. Lunsford, C.; Cale, Jr., A. D.; Ward, J. W.; Franko, B. V. and Jenkins, H.: 4-(b-substituted ethyl)-3, 3-diphenyl-2-pyrrolidinones. A new series of CNS stimulants. J. Med. Chem. 7:302 (1964).
2. Ward, J. W. and Franko, B. V.: A New Centrally Acting Agent (AHR-619) with Marked Respiratory Stimulating, Pressor, and “Awakening’’ Effects; Fed. Proc. 27:(2):325 (1962).
3. Funderburk, W. H.; Oliver, K. L. and Ward, J. W.: Electrophysiologic Analysis of the Site of Action of Doxapram Hydrochloride. J. Pharmacol. Exp. Ther. 151:3 (1966).
4. Funderburk, W. H.; Oliver, K. L.; Ward, J. W.: Cerebral Blood Flow Changes Due to Doxapram Hydrochloride (AHR-619); Fed. Proc. 22:(2):482 (Abstract) (1963).
5. Reports on File. Pharmacology Department, A. H. Robins Company.
6. Alphin, R. S. and Franko, B. V.: Inhibition and Stimulation of Gastric Secretions by Doxapram Hydrochloride (AHR-619); Fed. Proc. 22(2):662 (Abstract) (1963).
7. Kato, H. and Buckley, J. P.: Possible Sites of Action of the Respiratory Stimulant Effects of Doxapram Hydrochloride. J. Pharmacol. Exp. Ther. 144:260 (1964).
8. Bruce, R. B.; Pitts, J. E.; Pinchbeck, F. and Newman, J.: Excretion, Distribution, and Metabolism of Doxapram Hydrochloride. J. of Med. Chem. 8:157 (1965).
9. Woodard, G.; Ward, J. W. and Mann, G. T.: Safety Evaluation of the Respiratory Stimulant Doxapram Hydrochloride by Oral and Parenteral Administration to Laboratory Animals. Tox. and Appl. Pharmacol. 6:364 (1964).
10. Klemm, W. R.: Physiologic Responses to Equivalent Doses of Doxapram and Various Analeptic Combinations in Acute Barbiturate Narcosis in Dogs. Tox. and Appl. Pharmacol. 8:505 (1966).
11. Klemm, W. R.: Evaluation of Effectiveness of Doxapram and Various Analeptic Combinations in Dogs. J. Am. Vet. Med. Assoc. 148:894 (1966).
12. Jensen, E. C. and Klemm, W. R.: Clinical Evaluation of an Analeptic, Doxapram, in Dogs and Cats. J. Am. Vet. Med. Assoc. 150(5):516–525 (1967).
13. Polak, A. and Plum, F.: Comparison of New Analeptics in Barbiturate-Poisoned Animals. J. Pharmacol. Exp. Ther. 145:27 (1964).
14. Short, C. E.: Proc. American Animal Hospital Association, Washington, D. C., 1969.
| DOPRAM-V
doxapram hydrochloride injection |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Boehringer Ingelheim Vetmedica, Inc. (007134091) |
| Registrant - Boehringer Ingelheim Vetmedica, Inc. (007134091) |