IPRATROPIUM BROMIDE- ipratropium bromide spray
Mylan Specialty L.P.
----------
DESCRIPTION
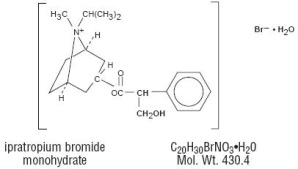
The active ingredient in Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is ipratropium bromide monohydrate. It is an anticholinergic agent chemically described as 8-azoniabicyclo (3.2.1) octane,3-(3-hydroxy-1-oxo-2-phenylpropoxy) -8-methyl-8-(1-methylethyl)-, bromide, monohydrate (endo,syn)-, (±)- : a synthetic quaternary ammonium compound, chemically related to atropine. Its structural formula is:
Ipratropium bromide is a white to off-white, crystalline substance. It is freely soluble in lower alcohols and water, existing in an ionized state in aqueous solutions, and relatively insoluble in non-polar media.
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is a metered-dose, manual pump spray unit which delivers 42 mcg ipratropium bromide (on an anhydrous basis) per spray (70 µL) in an isotonic, aqueous solution with pH adjusted to 4.7. It also contains benzalkonium chloride, edetate disodium, sodium chloride, sodium hydroxide, hydrochloric acid, and purified water. Each bottle contains 165 sprays.
CLINICAL PHARMACOLOGY
Mechanism of Action
Ipratropium bromide is an anticholinergic agent that inhibits vagally-mediated reflexes by antagonizing the action of acetylcholine at the cholinergic receptor. In humans, ipratropium bromide has anti-secretory properties and, when applied locally, inhibits secretions from the serous and seromucous glands lining the nasal mucosa. Ipratropium bromide is a quaternary amine that minimally crosses the nasal and gastrointestinal membrane and the blood-brain barrier, resulting in a reduction of the systemic anticholinergic effects (e.g., neurologic, ophthalmic, cardiovascular, and gastrointestinal effects) that are seen with tertiary anticholinergic amines.
Pharmacokinetics
Absorption
Ipratropium bromide is poorly absorbed into the systemic circulation following oral administration (2-3%). Less than 20% of an 84 mcg per nostril dose was absorbed from the nasal mucosa of normal volunteers, induced-cold adult volunteers, naturally-acquired common cold pediatric patients, or perennial rhinitis adult patients.
Distribution
Ipratropium bromide is minimally bound (0 to 9%in vitro) to plasma albumin and α1-acid glycoprotein. Its blood/plasma concentration ratio was estimated to be about 0.89. Studies in rats have shown that ipratropium bromide does not penetrate the blood-brain barrier.
Metabolism
Ipratropium bromide is partially metabolized to ester hydrolysis products, tropic acid and tropane. These metabolites appear to be inactive based on in vitro receptor affinity studies using rat brain tissue homogenates.
Elimination
After intravenous administration of 2 mg ipratropium bromide to 10 healthy volunteers, the terminal half-life of ipratropium was approximately 1.6 hours. The total body clearance and renal clearance were estimated to be 2,505 and 1,019 mL/min, respectively. The amount of the total dose excreted unchanged in the urine (Ae) within 24 hours was approximately one-half of the administered dose.
Pediatrics
Following administration of 84 mcg of ipratropium bromide per nostril three times a day in patients 5-18 years old (n=42) with a naturally acquired common cold, the mean amount of the total dose excreted unchanged in the urine of 7.8% was comparable to 84 mcg per nostril four times a day in an adult induced common cold population (n=22) of 7.3 to 8.1%. Plasma ipratropium concentrations were relatively low (ranging from undetectable up to 0.62 ng/mL). No correlation of the amount of the total dose excreted unchanged in the urine (Ae) with age or gender was observed in the pediatric population.
Special Populations
Gender does not appear to influence the absorption or excretion of nasally administered ipratropium bromide. The pharmacokinetics of ipratropium bromide have not been studied in patients with hepatic or renal insufficiency or in the elderly.
Drug-Drug Interactions
No specific pharmacokinetic studies were conducted to evaluate potential drug-drug interactions.
Pharmacodynamics
In two single-dose trials (n=17), doses up to 336 mcg of ipratropium bromide did not significantly affect pupillary diameter, heart rate, or systolic/diastolic blood pressure. Similarly, Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in adult patients (n=22) with induced colds, (84 mcg/nostril four times a day) and in pediatric patients (n=45) with naturally acquired common colds (84 mcg/nostril three times a day) had no significant effects on pupillary diameter, heart rate, or systolic/diastolic blood pressure.
Controlled clinical trials demonstrated that intranasal fluorocarbon-propelled ipratropium bromide does not alter physiologic nasal functions (e.g., sense of smell, ciliary beat frequency, mucociliary clearance, or the air conditioning capacity of the nose).
Clinical Trials
The clinical trials for Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) were conducted in patients with rhinorrhea associated with naturally occurring common colds. In two controlled four day comparisons of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) (84 mcg per nostril, administered three or four times daily; n=352) with its vehicle (n=351), there was a statistically significant reduction of rhinorrhea, as measured by both nasal discharge weight and the patients' subjective assessment of severity of rhinorrhea using a visual analog scale. These significant differences were evident within one hour following dosing. There was no effect of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) on degree of nasal congestion or sneezing. The response to Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) did not appear to be affected by age or gender. No controlled clinical trials directly compared the efficacy of three times daily versus four times daily treatment.
INDICATIONS AND USAGE
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is indicated for the symptomatic relief of rhinorrhea associated with the common cold for adults and children age 5 years and older. Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) does not relieve nasal congestion or sneezing associated with the common cold.
The safety and effectiveness of the use of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) beyond four days in patients with the common cold has not been established.
CONTRAINDICATIONS
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is contraindicated in patients with a history of hypersensitivity to atropine or its derivatives, or to any of the other ingredients.
WARNINGS
Immediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm and oropharyngeal edema.
PRECAUTIONS
General
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) should be used with caution in patients with narrow-angle glaucoma, prostatic hypertrophy or bladder neck obstruction, particularly if they are receiving an anticholinergic by another route. Cases of precipitation or worsening of narrow-angle glaucoma and acute eye pain have been reported with direct eye contact of ipratropium bromide administered by oral inhalation.
Information for Patients
Patients should be advised that temporary blurring of vision, precipitation or worsening of narrow-angle glaucoma or eye pain may result if Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) comes into direct contact with the eyes. Patients should be instructed to avoid spraying Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in or around the eyes. Patients who experience eye pain, blurred vision, excessive nasal dryness or episodes of nasal bleeding should be instructed to contact their doctor. Patients should be reminded to carefully read and follow the accompanyingPatient's Instructions for Use.
Drug Interactions
No controlled clinical trials were conducted to investigate potential drug-drug interactions. Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is minimally absorbed into the systemic circulation; nonetheless, there is some potential for an additive interaction with other concomitantly administered medications with anticholinergic properties, including ipratropium bromide for oral inhalation.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In two-year carcinogenicity studies in rats and mice, ipratropium bromide at oral doses up to 6 mg/kg (approximately 70 and 35 times the maximum recommended daily intranasal dose in adults, respectively, and approximately 45 and 25 times the maximum recommended daily intranasal dose in children, respectively, on a mg/m2 basis) showed no carcinogenic activity. Results of various mutagenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test, and chromosome aberration of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats was unaffected by ipratropium bromide at oral doses up to 50 mg/kg (approximately 600 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis). At an oral dose of 500 mg/kg (approximately 16,000 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis), ipratropium bromide produced a decrease in the conception rate.
Pregnancy
TERATOGENIC EFFECTS
Pregnancy Category B
Oral reproduction studies were performed at doses of 10 mg/kg in mice, 1,000 mg/kg in rats and 125 mg/kg in rabbits. These doses correspond, in each species respectively, to approximately 60, 12,000, and 3,000 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis. Inhalation reproduction studies were conducted in rats and rabbits at doses of 1.5 and 1.8 mg/kg, respectively, (approximately 20 and 45 times, respectively, the maximum recommended daily intranasal dose in adults on a mg/m2 basis). These studies demonstrated no evidence of teratogenic effects as a result of ipratropium bromide. At oral doses above 90 mg/kg in rats (approximately 1,100 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis) embryotoxicity was observed as increased resorption. This effect is not considered relevant to human use due to the large doses at which it was observed and the difference in route of administration. However, no adequate or well controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, ipratropium bromide should be used during pregnancy only if clearly needed.
Nursing Mothers
It is known that some ipratropium bromide is systemically absorbed following nasal administration; however, the portion which may be excreted in human milk is unknown. Although lipid-insoluble quaternary bases pass into breast milk, the minimal systemic absorption makes it unlikely that ipratropium bromide would reach the infant in an amount sufficient to cause a clinical effect. However, because many drugs are excreted in human milk, caution should be exercised when Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is administered to a nursing woman.
Pediatric Use
The safety of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) at a dose of two sprays (84 mcg) per nostril three times a day (total dose 504 mcg/day) for two to four days has been demonstrated in two clinical trials involving 362 pediatric patients 5-11 years of age with naturally acquired common colds. In this pediatric population, Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) had an adverse event profile similar to that observed in adolescent and adult patients.
When Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) was concomitantly administered with an oral decongestant (pseudoephedrine HCl) in 122 children ages 5-12 years, and concomitantly administered with an oral decongestant/antihistamine combination (pseudoephedrine HCl/chlorpheniramine maleate) in 123 children ages 5-12 years, adverse event profiles were similar to ipratropium bromide alone. The effectiveness of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) for the treatment of rhinorrhea associated with the common cold in this pediatric age group is based on extrapolation of the demonstrated efficacy of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in adolescents and adults with the condition and the likelihood that the disease course, pathophysiology, and the drug's effect are substantially similar to that of adults. The recommended dose for common cold for the pediatric population is based on cross-study comparisons of the efficacy of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in adult and pediatric patients and on its safety profile in both adults and pediatric patients.
The safety and effectiveness of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in pediatric patients under 5 years of age have not been established.
ADVERSE REACTIONS
Adverse reaction information on Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in patients with the common cold was derived from two multicenter, vehicle-controlled clinical trials involving 1,276 patients (195 patients on Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray), 352 patients on Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray), 189 patients on Ipratropium Bromide Nasal Spray 0.12%, 351 patients on vehicle and 189 patients receiving no treatment).
The following table shows adverse events reported for patients who received Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) at the recommended dose of 84 mcg per nostril, or vehicle, administered three or four times daily, where the incidence is 1% or greater in the Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) group and higher in the Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) group than in the vehicle group.
| % of Patients Reporting Events* | ||
|---|---|---|
| Ipratropium Bromide
Nasal Solution 0.06% (Nasal Spray) (N=352) | Vehicle
Control (N=351) |
|
|
||
|
Epistaxis† |
8.2% |
2.3% |
|
Nasal Dryness |
4.8% |
2.8% |
|
Dry Mouth/Throat |
1.4% |
0.3% |
|
Nasal Congestion |
1.1% |
0.0% |
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) was well tolerated by most patients. The most frequently reported adverse events were transient episodes of nasal dryness or epistaxis. The majority of these adverse events (96%) were mild or moderate in nature, none was considered serious, and none resulted in hospitalization. No patient required treatment for nasal dryness, and only three patients (<1%) required treatment for epistaxis, which consisted of local application of pressure or a moisturizing agent (e.g., petroleum jelly). No patient receiving Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) was discontinued from the trial due to either nasal dryness or bleeding.
Adverse events reported by less than 1% of the patients receiving Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) during the controlled clinical trials that are potentially related to Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) local effects or systemic anticholinergic effects include: taste perversion, nasal burning, conjunctivitis, coughing, dizziness, hoarseness, palpitation, pharyngitis, tachycardia, thirst, tinnitus and blurred vision.
No controlled trial was conducted to address the relative incidence of adverse events for three times daily versus four times daily therapy.
Additional anticholinergic effects noted with other ipratropium bromide dosage forms (Ipratropium Bromide Inhalation Solution, Ipratropium Bromide Inhalation Aerosol and Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray)) include: precipitation or worsening of narrow-angle glaucoma, urinary retention, prostate disorders, constipation and bowel obstruction.
There were no reports of allergic-type reactions in the controlled clinical trials. Allergic-type reactions such as skin rash, angioedema of the tongue, lips and face, urticaria, laryngospasm and anaphylactic reactions have been reported with other ipratropium bromide products.
OVERDOSAGE
Acute overdosage by intranasal administration is unlikely since ipratropium bromide is not well absorbed systemically after intranasal or oral administration. Following administration of a 20 mg oral dose (equivalent to ingesting more than two bottles of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray)) to 10 male volunteers, no change in heart rate or blood pressure was noted. Following a 2 mg intravenous infusion over 15 minutes to the same 10 male volunteers, plasma ipratropium concentrations of 22-45 ng/mL were observed (>100 times the concentrations observed following intranasal administration). Following intravenous infusion these 10 volunteers had a mean increase of heart rate of 50 bpm and less than 20 mm Hg change in systolic or diastolic blood pressure at the time of peak ipratropium levels.
Oral median lethal doses of ipratropium bromide were greater than 1,000 mg/kg in mice (approximately 6,000 and 3,800 times the maximum recommended daily intranasal dose in adults and children, respectively, on a mg/m2 basis) 1,700 mg/kg in rats (approximately 21,000 and 13,000 times the maximum recommended daily intranasal dose in adults and children, respectively, on a mg/m2 basis) and 400 mg/kg in dogs (approximately 16,000 and 10,000 times the maximum recommended daily intranasal dose in adults and children, respectively, on a mg/m2 basis).
DOSAGE AND ADMINISTRATION
The recommended dose of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is two sprays (84 mcg) per nostril three or four times daily (total dose 504 to 672 mcg/day) in adults and children age 12 years and older. Optimum dosage varies with response of the individual patient. The recommended dose of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) for children age 5-11 years is two sprays (84 mcg) per nostril three times daily (total dose of 504 mcg/day).
The safety and effectiveness of the use of Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) beyond four days in patients with the common cold have not been established.
Initial pump priming requires seven sprays of the pump. If used regularly as recommended, no further priming is required. If not used for more than 24 hours, the pump will require two sprays, or if not used for more than seven days, the pump will require seven sprays to reprime.
HOW SUPPLIED
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is supplied in a white high density polyethylene (HDPE) bottle fitted with a metered Nasal Solution pump, a purple safety clip to prevent accidental discharge of the spray, and a clear plastic dust cap. It contains 16.6 g of product formulation, 165 sprays, each delivering 42 mcg of ipratropium bromide per spray (70 µL), or 10 days of therapy at the maximum recommended dose (two sprays per nostril four times a day).
1 bottle per carton NDC 49502-786-15
Store tightly closed between 59°F (15°C) and 86°F (30°C). Avoid freezing. Keep out of reach of children. Do not spray in the eyes.
Patients should be reminded to read and follow the accompanying Patient's Instructions for Use, which should be dispensed with the product.
Rx only.
DEY
Napa, CA 94558
03-561-03
Printed: April 2003
Patient's Instructions for Use
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is indicated for the symptomatic relief of rhinorrhea (runny nose) associated with the common cold for adults and children age 5 years and older. Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) does not relieve nasal congestion or sneezing associated with the common cold. Do not use Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) for longer than four days unless instructed by your physician.
Read complete instructions carefully and use only as directed.
To Use:
- 1.
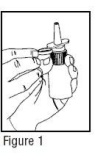
- Remove the clear plastic dust cap and the purple safety clip from the Nasal Solution pump (Figure 1). The safety clip prevents the accidental discharge of the spray in your pocket or purse.
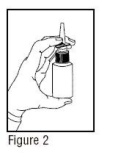
- 2.
- The Nasal Solution pump must be primed before Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is used for the first time. To prime the pump, hold the bottle with your thumb at the base and your index and middle fingers on the white shoulder area. Make sure the bottle points upright and away from your eyes. Press your thumb firmly and quickly against the bottle seven times (Figure 2). The pump is now primed and can be used. Your pump should not have to be reprimed unless you have not used the medication for more than 24 hours; repriming the pump will only require two sprays. If you have not used your Nasal Solution for more than seven days, repriming the pump will require seven sprays.
- 3.
- Before using Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray), blow your nose gently to clear your nostrils if necessary.
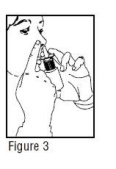
- 4.
- Close one nostril by gently placing your finger against the side of your nose, tilt your head slightly forward and, keeping the bottle upright, insert the nasal tip into the other nostril (Figure 3). Point the tip toward the back and outer side of the nose.
- 5.
- Press firmly and quickly upwards with the thumb at the base while holding the white shoulder portion of the pump between your index and middle fingers. Following each spray, sniff deeply and breathe out through your mouth.
- 6.
- After spraying the nostril and removing the unit, tilt your head backwards for a few seconds to let the spray spread over the back of the nose.
- 7.
- Repeat steps 4 through 6 in the same nostril.
- 8.
- Repeat steps 4 through 7 in the other nostril (i.e., two sprays per nostril).
- 9.
- Replace the clear plastic dust cap and safety clip.
- 10.
- You should not take extra doses or stop using Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) without consulting your physician.
To Clean:
If the nasal tip becomes clogged, remove the clear plastic dust cap and safety clip. Hold the nasal tip under running, warm tap water (Figure 4) for about a minute. Dry the nasal tip, reprime the Nasal Solution pump (step 2 above), and replace the plastic dust cap and safety clip.
Caution:
Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) is intended to relieve your rhinorrhea (runny nose) with regular use. It is therefore important that you use Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) as prescribed by your physician. For most patients, some improvement in runny nose is usually apparent following the first dose of treatment with Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray). Do not use Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) for longer than four days unless instructed by your physician.
Do not spray Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in your eyes. Should this occur, immediately flush your eye with cool tap water for several minutes. If you accidentally spray Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray) in your eyes, you may experience a temporary blurring of vision, increased sensitivity to light, and widening of the pupil which, may last a few hours. Should eye pain or blurred vision occur, contact your doctor.
Should you experience excessive nasal dryness or episodes of nasal bleeding contact your doctor.
If you have glaucoma or difficulty urinating due to an enlargement of the prostate, be sure to tell your physician prior to using Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray).
If you are pregnant or you are breast feeding your baby, be sure to tell your physician prior to using Ipratropium Bromide Nasal Solution 0.06% (Nasal Spray).
Storage:
Store tightly closed between 59°F (15°C) and 86°F (30°C). Avoid freezing. Keep out of reach of children.
Manufactured by
Dey
Napa, CA 94558
03-561-03
April 2003
| IPRATROPIUM BROMIDE
ipratropium bromide spray |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Mylan Specialty L.P. (194775557) |