EAR MITE RELIEF PLUS PAIN RELIEF- lidocaine liquid
Swabplus Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
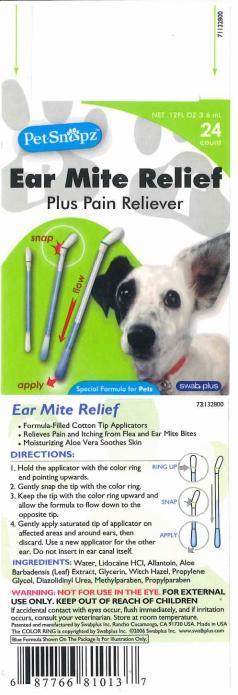
Ear Mite Relief
Formula-Filled Cotton Tip Applicators.
Relieves Pain and Itching fromFlea and Ear Mite Bites.
Moisturizing Aloe Vera Soothes Skin.
DIRECTIONS
1. Hold the applicator with the color ring end pointing upwards.
2. Gently snap the tip with the color ring.
3. Keep the tip with the color ring upward and allow the formula to flow down to the opposite tip.
4. Gently apply saturated tip of applicator on affected areas and around ears, then discard. Use a new applicator for the other ear. Do not insert in ear canal itself.
INACTIVE INGREDIENTS
Water, Allantoin, Glycerin, Aloe Vera Leaf, Witch Hazel, Propylene Glycol, Diazolidinyl Urea, Methylparaben, Propyparaben.
STORAGE AND HANDLING
If accidental contact with eyes occur, flush immediately, and if irritation occurs, consult your veterinarian.
Store at room temperature.
| EAR MITE RELIEF PLUS PAIN RELIEF
lidocaine liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Swabplus Inc. (876441549) |
| Registrant - Swabplus Inc. (876441549) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Swabplus Inc. | 876441549 | manufacture, api manufacture, relabel, repack | |